Cyanoacrylate Adhesives

Cyanoacrylate Adhesives
Cyanoacrylate is a general term for a category of fast and very strong adhesives that have numerous applications in household, medical, and industrial uses. Cyanoacrylate adhesives have a short shelf life, remaining usable for about one month after opening and up to one year after production. They are also slightly toxic. These adhesives are sometimes known as instant adhesives or drop adhesives, and those with an active spray type are called “one-two-three” adhesives. In English, the trademark “Super Glue” is commonly associated with this type of adhesive.
Cyanoacrylates are liquid monomers that polymerize when placed between two surfaces, forming a thin film. A small amount of moisture on the surface can cause the adhesive to dry very quickly (in less than 2 seconds). In other words, cyanoacrylate adhesive only interacts with a surface in the presence of slight moisture; if this adhesive is in a moisture-free environment, it cannot create any bond.

There are various types of cyanoacrylates, differing in their chemical base and concentration.
Ethyl Ester Base: Due to the size of the molecules and the anchor points created with space between them, this type creates high elasticity and strong bonds, suitable for bonding rubber and plastics.
Alkoxy Ethyl Base: These adhesives have good flexibility due to their specific base characteristics and similar molecular structure. Another notable feature of these products is their low odor, making them easy to use, especially in production lines. These adhesives have reduced sensitivity to moisture after drying and are typically used where the white color of the adhesive after drying should not be visually apparent.
Methyl Ester Base: Due to the smaller molecular structure and closer anchor points, these adhesives have less flexibility after drying. Products with this base are suitable for bonding metals.
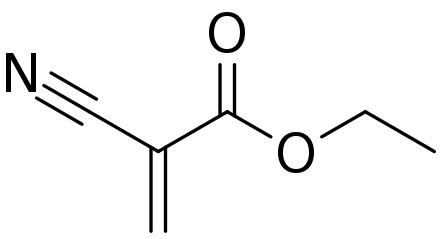
The most common monomer is ethyl 2-cyanoacrylate. Several related esters are recognized. Generally, C=C groups quickly undergo polymerization in the presence of water, forming long and strong chains that bond surfaces together. Since the presence of moisture causes the adhesive to harden, exposure to normal moisture levels in the air leads to the formation of a thin layer within seconds, which significantly slows down the reaction. Therefore, cyanoacrylates are applied as thin coatings—rather than thick ones—to ensure that the reaction occurs quickly for bonding.
General Characteristics
This substance is a liquid composed of cyanoacrylate ester monomers. Methyl 2-cyanoacrylates have a molecular weight of 111.1 grams per mole, a flash point of 79 degrees Celsius (174 degrees Fahrenheit), and a density of 1.1 grams per mole. Ethyl 2-cyanoacrylates weigh about 125 grams per mole and have a flash point above 75 degrees Celsius (167 degrees Fahrenheit). To improve the usability of the adhesive, it has recently been combined with materials such as fumed silica to achieve a sticky or gel-like state. Various additives are added to this resin to enhance its shear strength and create a shock-resistant composite, such as rubber.
In general, acryl groups rapidly undergo chain polymerization processes in the presence of water (especially hydroxyl ions), producing long and strong chains that connect bonding surfaces. Since the presence of moisture causes the adhesive to harden and set, exposure to a slight amount of moisture in the air creates a thin layer of these materials within seconds, ultimately slowing the reaction. Cyanoacrylates are thus used as thin coatings to ensure that the bonding speed is not compromised. The shelf life of this adhesive is very short; it takes about one month after production (if unopened) for the adhesive to become unusable. The reaction of this substance with moisture causes an opened or used container to become unusable more quickly than unopened types. To prevent such reactions and extend the lifespan of this substance, the adhesive should be kept in packages containing silica gel desiccants. Another method involves using subcutaneous needles at the entrance of adhesive tubes, preventing moisture from entering by sticking to the needle. The polymerization reaction of this substance is also temperature-dependent, and if exposed to temperatures below the freezing point of water or 0 degrees Celsius (32 degrees Fahrenheit), this reaction halts. Therefore, it can also be stored in a freezer.
Note: Cyanoacrylate adhesives have a weak bond with smooth surfaces, making them easy to separate. A good example of this fact is that cyanoacrylates may be removed from human skin using abrasive materials (such as sugar or sandpaper).
Cosmetic Uses
Cyanoacrylate is used in the beauty industry as eyelash extension glue or “nail glue” for certain artificial nail enhancers, such as nail tips and nail coverings.
Medical Uses
Tissue adhesives have long been used in the beauty and medical industries and in first aid. Histocryl glue, produced by B. Braun in Germany, is one of the most well-known and high-quality adhesives available in the medical market worldwide. Today, cyanoacrylate glue is widely used in a range of surgical procedures. Cyanoacrylate guarantees high-quality adhesion and biocompatibility compared to other types of adhesives. Due to the polymerization process, when cyanoacrylate glue comes into contact with blood or tissue fluid, it forms a strong and robust coating.
Applications of Cyanoacrylate Adhesive
Urinary fistula, gastrointestinal fistula, lymphocele, endoscopic procedures, laparoscopic procedures, bladder tumor removal, cutaneous ureteral fistula, partial nephrectomy, tumor excision, varicocele, arteriovenous fistula, hypospadias, priapism, skin gap closure, pediatric urology, mesh fixation, and more.
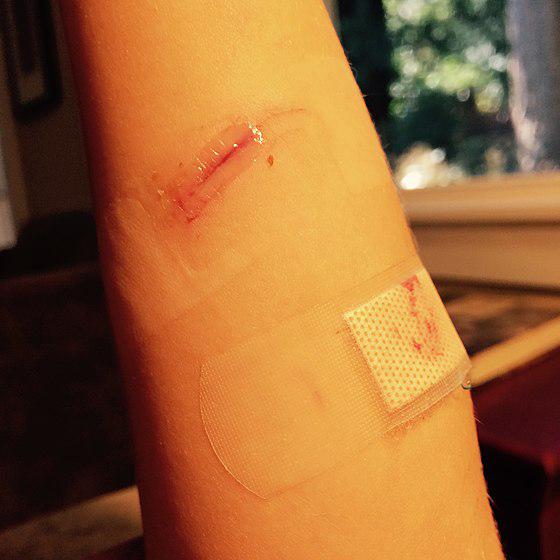
Skin Injuries
Cyanoacrylate adhesives may adhere to parts of the body, causing injury when removed along with portions of skin. However, without force, the adhesive will detach from the skin on its own after a period (maximum four days). In cases where the eyelid is glued, particularly if it contacts the eyeball, medical professionals must be involved.
Toxicity
Heat causes the depolymerization of baked polymers and produces gaseous products that are highly irritating. They polymerize and become inert immediately upon contact with moisture in membranes. These risks can be minimized by using cyanoacrylate in well-ventilated areas. Approximately 5% of the population may develop sensitivity to cyanoacrylate vapors after repeated exposure. Cyanoacrylate may also act as a skin irritant, causing allergic skin reactions. In rare cases, inhalation may cause asthma. There is no specific measurement of toxicity for cyanoacrylate adhesives since many adhesives contain various cyanoacrylate formulas. The compound 2-octyl cyanoacrylate is much slower to decompose due to its longer organic backbone (a series of carbon molecules with covalent bonds) and does not reach the threshold of tissue toxicity. Given the toxicity of ethyl cyanoacrylate, the use of 2-octyl cyanoacrylate is preferred for sutures.
Solvents
Acetone, found in nail polish remover, is one of the most accessible solvents for softening cyanoacrylate and separating bonded pieces. Other solvents include nitromethane and dimethyl sulfoxide. Gamma butyrolactone can also be used to remove dried cyanoacrylate.

Important Usage Notes
When using cyanoacrylate adhesive, it should be applied as a thin layer on the desired surface. A critical point during use is ensuring that the surface is clean and dry; if a thick layer is applied, the bonding will not work effectively. Ensure adequate ventilation in the workspace when using cyanoacrylate adhesives, as adhesive vapors will be present in the bonding area. Since cyanoacrylate drop adhesives have high adhesion to various materials—especially human skin—it is recommended to wear gloves during use. These adhesives also react quickly and exothermically with cotton, wool, leather, etc., so caution should be exercised during application.
Finally, it’s essential to keep in mind that numerous factors influence the choice of a suitable cyanoacrylate adhesive for your application. Given the wide variety of these adhesives, it is crucial to consult our expert before purchasing to ensure you select the best drop adhesive based on the surface materials and your specific application conditions.













