How Adhesives Work

How Adhesives Work
To better understand how adhesives bond to surfaces, it’s important to know the mechanisms of adhesion. There are four recognized methods for the adhesion of adhesives and tapes to surfaces:
1. Chemical Adhesion Mechanism
The chemical adhesion mechanism occurs when the polymer adhesive penetrates the substrate through molecular contact. This is the strongest adhesion mechanism, involving molecular bonds between functional groups in the adhesive and the molecules of the substrate. This mechanism provides the structural strength of many adhesives, such as epoxy adhesives for bonding aluminum.
2. Mechanical Locking Mechanism
The mechanical locking mechanism happens when, during the adhesion process, the adhesive penetrates the pores of the substrate. Locking creates a physical barrier to the propagation of cracks at the joint. It also increases the surface area, enhancing the bond between the adhesive and the substrate. Liquid adhesives flow into the substrate as they dry and cure, and adhesive bonds gradually extend over time, increasing their strength.
3. Diffusion Mechanism
The diffusion mechanism occurs when the polymer adhesive is able to penetrate and spread into the polymer substrate. The points of attachment of the adhesive’s polymer chains engage with and merge into the substrate. This mechanism is common in the assembly of materials with low surface energy, such as polypropylene.
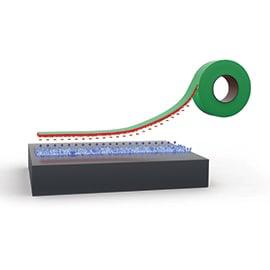
4. Electrostatic Adhesion Mechanism
The electrostatic adhesion mechanism happens when the surface of the adhesive interacts with the substrate, attracting with opposite charges, similar to how tapes adhere. This phenomenon is often observed when trying to stick a box or gift wrapping together.













